Speeding Up Earth’s Natural CO2 Mineralization Cycle Can Recover All of the CO2 from Fossil Fuels
The Carbon Storage Problem
We’ve got all these great solutions capturing carbon, typically from air or industrial emitters. But capture is only half of the problem. What do we do with all that captured carbon?
The duration of carbon storage is critical to assessing the life cycle of CO2-derived products and storage capabilities. Synthetic fuels derived from captured CO2 are typically recombusted within one year of being captured. Therefore, synthetic fuels are not a viable long-term storage solution. Rather, geologic carbon storage is a widely accepted long-term solution which has a global technical potential of at least 2,000 Gt of CO2, IPCC 2018. Here, carbon is injected into underground reservoirs, mostly for enhanced oil recovery for oil and gas.
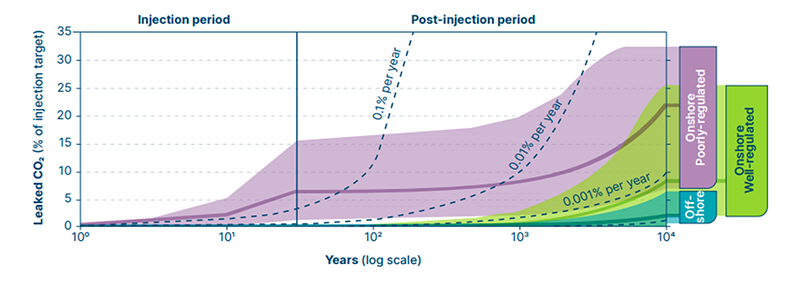
A typical well has a potential capacity of 1 Mt/year. However, this solution, although common, is known to result in CO2 leakage. Ideally, less than 1% leakage should be expected over a 1,000-year period. However, continuous leakage and well blowouts are known to lead to closures and increased project costs. In the U.S. alone, if all of the active and abandoned oil and gas wells leaked at the average leakage rate, leakage could be upwards of 60M tons of CO2 per year.
Potential CO2 Leakage by Form and Quality of Storage
Is There a Better Way to Store Underground?
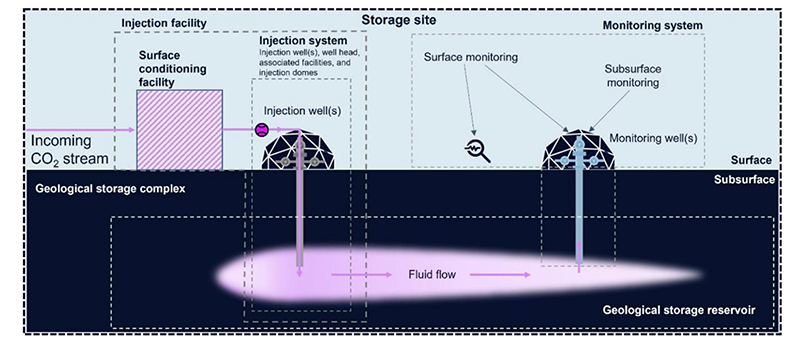
Subsurface mineralization stores CO2 in solid form, generally as a carbonate mineral in either in-situ, ex-situ, or surficial operations. For in-situ operations, CO2 and water are injected underground to create a calcium carbonate derivative that stores the CO2 when reacted with calcium-/magnesium-bearing minerals like mafic and ultramafic rocks that are globally abundant. Water is sourced from the same reservoir in which the injection takes place or seawater may be used.
Solidification can take up to two years to form a stable mineral before CO2 is permanently sequestered for millennia. Leakage is so low that it’s essentially eliminated with instant solubility of dissolving CO2 in water. But lack of effective monitoring techniques for subsurface and surface systems exist to keep track of gas and water leakage while the CO2 solidifies. Still, no long-term monitoring is needed.
Subsurface Flowsheet
How Much Can Be Stored?
Storage has the most potential with mafic or ultramafic (basalt, igneous, or magma) rocks because they are highly reactive and contain the metals needed to permanently immobilize CO2. The theoretical storage capacity exceeds the total CO2 stemming from the burning of all fossil fuel-derived carbon on Earth. Globally, the discovered storage capacity is upwards of 250 GT of CO2 in on-land basalts and up to 100 GT in submarine basalts (National Academies of Sciences Engineering Medicine, 2019).
Other critical factors such as the availability of water or permeability of the bedrock can vary greatly between regions. Basaltic rocks vary in terms of how fractured and porous they are, which can impact the total storage space for the mineralized CO2. For example, many basalts in the U.S. do not have potential for storage due to their shallow depth, closed fractures, and high probability of fault reactivation. Other reactive rocks such as andesites, peridotites, breccias and sedimentary formations containing calcium, magnesium, and iron-rich silicate minerals may also be feasible.
Technical and Economic Considerations
It’s difficult to estimate the storage capacity of a well in the long-term partly because there exists a maximum rate of injectivity for a given reservoir. The rate of mineralization depends on the amount of dissolved CO2, the presence of divalent ions in the host rock, and the alkalinity of the solution it’s dissolved in. This step is perhaps the most limiting as researchers are trying to achieve more rapid carbonation acceleration. Moreover, utilization of heat that is generated during the process is of interest. Still, in-situ mineralization does not require additional facilities, mining, or transportation of reactants or minerals.
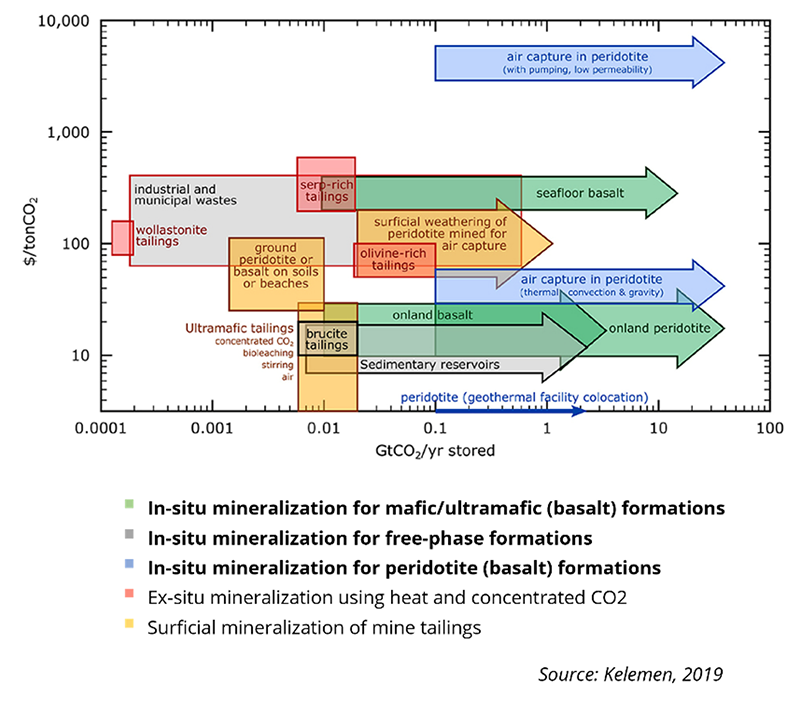
Basalts are of main consideration since over 90% mineralizes within just a few months. Researchers believe basalt systems may be self-sealing where mineralization is common at “dead-ends” thus containing itself. At 30 bar pressure and 20°C, approximately 22 mt of water is required per ton of CO2 that costs $10-$40 per ton. Carbfix’s pilot facilities cost approximately $10M-$20M per year or $25 per ton of soluble gas stored using existing infrastructure at a large geothermal facility. Free-phase CO2-based mineralization typically runs $5M per well. Costs are strongly correlated with permeability, where low permeability incurs higher costs due to larger water volume requirements. But there’s a negative correlation between cost and CO2 content thus carbon capture is attractive to increase CO2 purity.
CO2 Mineralization Cost + Volume Comparison
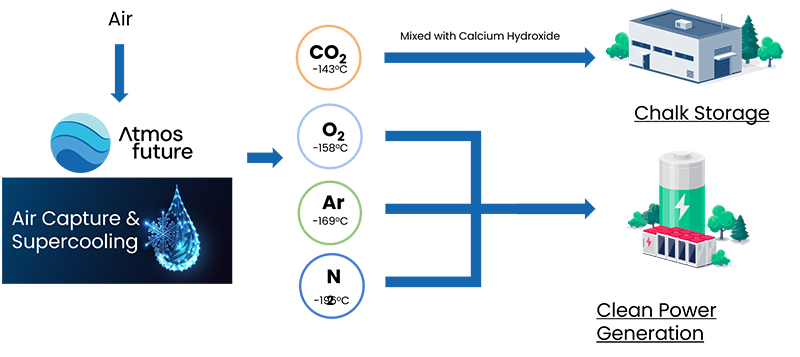
This is why innovator, Atmosfuture, combines its fanless, cryogenic-based REVFRACC system (REVerse FRActionation Carbon Capture), a Direct Air Capture (DAC) solution, with CO2 utilization. Once captured, CO2 is then mixed with calcium hydroxide to create chalk in an exothermic reaction. The resulting chalk suspension can be used to pump into depleted oil and gas wells. The chalk method can be used to reconstitute open chalk mines which are depleted or sold as part of a circular economy in building.
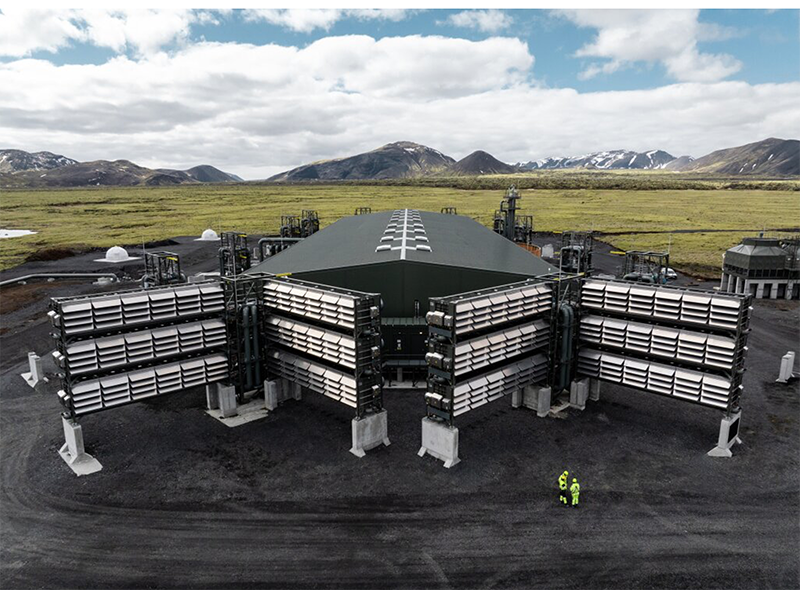
This draws on the momentum of leading innovators like Carbfix, known for its subsurface mineralization solution that captures carbon from point source emitters or by DAC near promising rock formations, like for geothermal projects. Climeworks launched its largest project, Mammoth, in Hellisheiði, Iceland in 2022. It’s a DAC plant that will have an annual capture capacity of 36,000 tons of which Carbfix will be responsible for storing the CO2 underground in basaltic rocks. It’s expected to begin operations this year.
Project Mammoth
What’s Holding the Industry Back From Scaling More Rapidly?
Currently, there exists wide technical knowledge gaps that must be addressed at field scale. Some challenges with water remain to be tackled like utilization of seawater over freshwater. Fortunately, there have been a few projects that have successfully demonstrated subsurface mineralization, particularly by Carbfix. Future projects will need to co-locate where large supplies of CO2 and abundant basaltic rocks are available like geothermal reservoirs. Because it’s generally unlikely that the best suited rock formations will be near industrial emissions, DAC presents a positive opportunity to maximize capture and storage. Utilization of other rock formations may also help accelerate the rate of mineralization such as ultramafic rocks, but further studies are still needed.
Regulations will need to be relaxed once it’s understood that subsurface mineralization is more secure than those systems utilized by the oil and gas industry with supercritical (liquid) CO2. Likewise, education is needed to ensure the public that these systems will not harm local environments — the most significant concerns being human-induced tremors. Despite these hurdles, the potential of subsurface mineralization to provide a safe and permanent solution for carbon storage only warrants rapid deployment.



